Entry 1 of 2. The primary difference between haloalkanes and haloarenes is that haloalkanes are derived from open-chain hydrocarbons alkanes whereas haloarenes are derived from aromatic hydrocarbons. What is the definition of halogen in chemistry.
What Is The Definition Of Halogen In Chemistry, The halogens show trends in their physical and chemical properties. We call it also Alkyl Halides. A halogen is one of a group of chemical elements that includes chlorine fluorine and iodine. Halogens do not exist in the elemental form in nature.
 Halogen Meaning Youtube From youtube.com
Halogen Meaning Youtube From youtube.com
It is so reactive it even forms compounds with Kr Xe and Rn elements that were once thought to be inert. These are also known as alkyl halides and aryl halides respectively. Haloalkanes and haloarenes are the hydrocarbons in which one or more hydrogen atoms have been replaced with halogen atoms. Halogen lamps are illuminated by bulbs that contain a halogen and an inert gas.
If the other member of the bond is a hydrogen atom bonded to a more electronegative element I dont see have the halogen atom could be an acceptor of electron density.
Read another article:
The other name of Haloalkane is Alkyl Halides. Halogens Halogens Halogens are nonmetals. It is so reactive it even forms compounds with Kr Xe and Rn elements that were once thought to be inert. It can donate a pair of electrons to the Lewis acid H to form H 2 O. Elements in the halogen group have seven electrons in their outer shells giving them many unique properties.
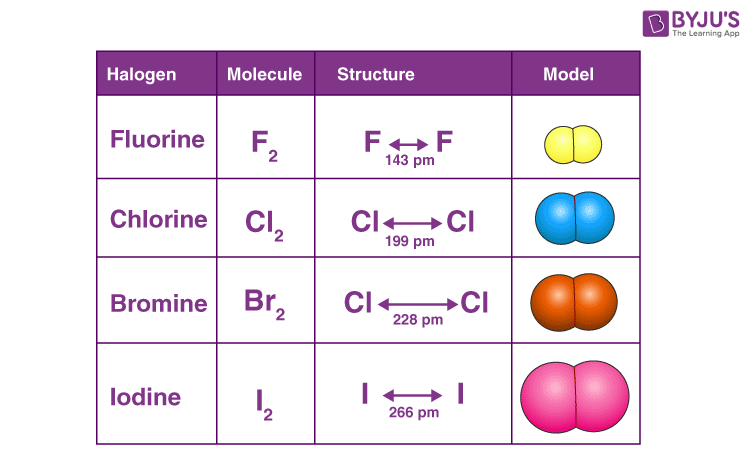 Source: byjus.com
Source: byjus.com
The halogens show trends in their physical and chemical properties. In chemistry halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. It is so reactive it even forms compounds with Kr Xe and Rn elements that were once thought to be inert. It can donate a pair of electrons to the Lewis acid H to form H 2 O. Halogens Definition Uses Compounds Properties Of Halogens.
 Source: youtube.com
Source: youtube.com
There are five halogens in the periodic table of chemical elements. This kind of conversion is in fact so common that a comprehensive overview is challenging. We will discuss the definition of haloalkanes and haloarenes examples of haloalkanes and haloarenes difference between haloalkanes and haloarenes and their classification in this article. To the carbon atom that is bonded to the halogen. Group 7 The Halogens Properties Of Matter Chemistry Fuseschool Youtube.
 Source: docbrown.info
Source: docbrown.info
Physical States of Halogens Halogens represents all of the three familiar states of matter. The halogens are all highly reactive which means theyre quick to form bonds with other elements. The graph shows the melting and boiling points of the first four group 7 elements. They are placed in the vertical column second from the right in the periodic table. Group 7 Halogens Fluorine Chlorine Bromine Iodine Physical Properties Balanced Equations Chemical Reactions Balanced Gcse Chemistry Revision Notes Ks4 Science Igcse O Level.
 Source: scienceclarified.com
Source: scienceclarified.com
The graph shows the melting and boiling points of the first four group 7 elements. Halogens are often used in lighting and heating devices. Halogens are often used in lighting and heating devices. It is so reactive it even forms compounds with Kr Xe and Rn elements that were once thought to be inert. Halogens Humans Body Used Water Process Earth Life Plants.
 Source: chemistryexplained.com
Source: chemistryexplained.com
We will discuss the definition of haloalkanes and haloarenes examples of haloalkanes and haloarenes difference between haloalkanes and haloarenes and their classification in this article. Haloalkanes - It is an aliphatic hydrocarbon compound but in it one or more hydrogen atoms is replaced by one or more halogen atoms called Haloalkanes. Haloalkanes and Haloarenes are halogen derivatives of Alkanes and Arenes. Scientific definitions for halogen halogen hăl ə-jən Any of a group of five nonmetallic elements with similar properties. Organic Halogen Compounds Chemistry Encyclopedia Reaction Elements Examples Gas Number Atom Synthesis Reactivity.
 Source: youtube.com
Source: youtube.com
It can donate a pair of electrons to the Lewis acid H to form H 2 O. Chlorine bromine and iodine are the three common Group 7. What elements are halogens. The halogens are a group of elements in the periodic table. Halogens Song Periodic Table For Kids Youtube.
 Source: msrblog.com
Source: msrblog.com
255 Hydrolysis Breaking up molecules by reaction with water. It is so reactive it even forms compounds with Kr Xe and Rn elements that were once thought to be inert. Halide-containing compounds are pervasive making this type of transformation important eg. The other name of Haloalkane is Alkyl Halides. Halogen Msrblog.
 Source: docbrown.info
Source: docbrown.info
These are also known as alkyl halides and aryl halides respectively. Iodine and astatine are solids. Of or relating to the group of elements that are halogens. The graph shows the melting and boiling points of the first four group 7 elements. Group 7 Halogens Fluorine Chlorine Bromine Iodine Physical Properties Balanced Equations Chemical Reactions Balanced Gcse Chemistry Revision Notes Ks4 Science Igcse O Level.
 Source: youtube.com
Source: youtube.com
A member of a group of five. The halogens are a group of elements in the periodic table. The primary difference between haloalkanes and haloarenes is that haloalkanes are derived from open-chain hydrocarbons alkanes whereas haloarenes are derived from aromatic hydrocarbons. A halogen is one of a group of chemical elements that includes chlorine fluorine and iodine. Halogen Meaning Youtube.
 Source: chemistrylearner.com
Source: chemistrylearner.com
A member of a group of five particular chemical elements. 254 Reflux Repeated boiling and condensing of a reaction mixture. Halogens are very reactive the reactivity decreases from fluorine to astatine. Water hydrogen sulfide and ammonia are all nucleophiles. Halogens Chemistry Learner.
 Source: chubbyrevision.weebly.com
Source: chubbyrevision.weebly.com
This kind of conversion is in fact so common that a comprehensive overview is challenging. The halogens while not nucleophilic in diatomic form eg I 2 are nucleophiles as anions eg I -. This kind of conversion is in fact so common that a comprehensive overview is challenging. The halogens are fluorine chlorine bromine iodine and astatine. Chem Grp7 Chubby Revision As Level.
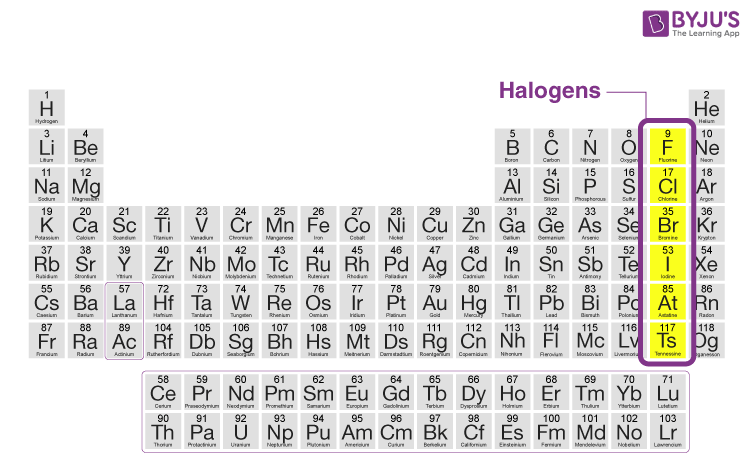 Source: byjus.com
Source: byjus.com
The Group 7 elements are called the halogens. A member of a group of five particular chemical elements. The graph shows the melting and boiling points of the first four group 7 elements. The halogens are a group of elements in the periodic table. Halogens Definition Uses Compounds Properties Of Halogens.
 Source: chem4kids.com
Source: chem4kids.com
Astatine is placed below iodine in group 7. It can donate a pair of electrons to the Lewis acid H to form H 2 O. Halogens Halogens Halogens are nonmetals. They are located to the right of the other nonmetals and to the left of the noble gases. Chem4kids Com Elements Periodic Table Halogens.
 Source: youtube.com
Source: youtube.com
The halogens are fluorine chlorine bromine iodine and astatine. The Group 7 elements are called the halogens. Water hydrogen sulfide and ammonia are all nucleophiles. Physical States of Halogens Halogens represents all of the three familiar states of matter. What Is Halogen What Does Halogen Mean Halogen Meaning Definition Explanation Youtube.
 Source: britannica.com
Source: britannica.com
Halogens Halogens Halogens are nonmetals. The graph shows the melting and boiling points of the first four group 7 elements. At room temperature fluorine and chlorine are gases and bromine is a liquid. Water hydrogen sulfide and ammonia are all nucleophiles. Halogen Elements Examples Properties Uses Facts Britannica.







